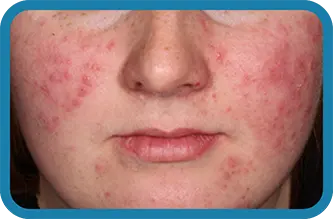
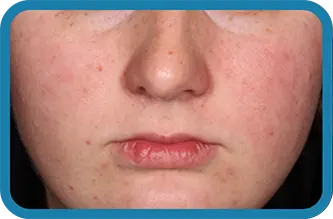
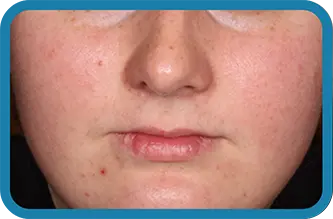
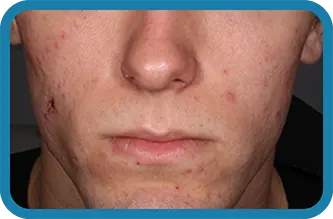
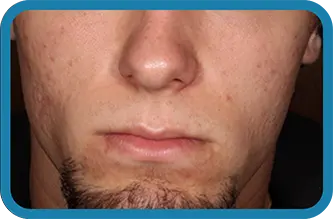
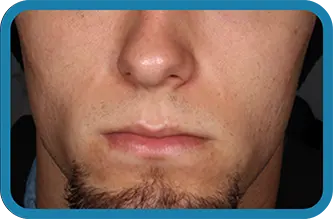
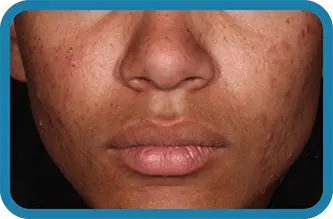
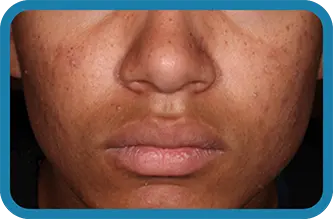
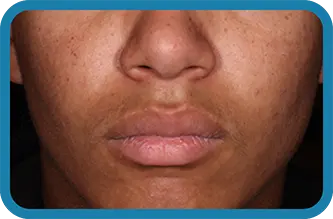
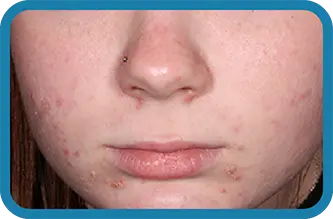
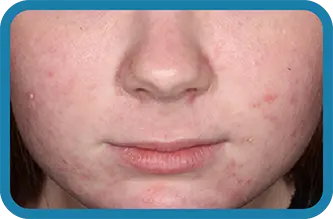
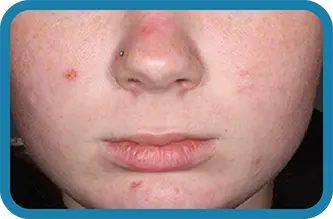
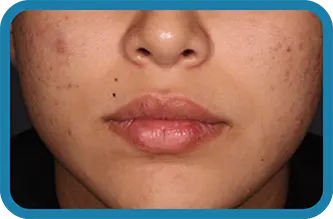
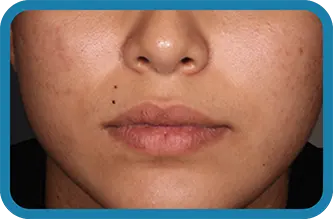
See the difference in real people.
Noticeable results as early as 3 weeks.1
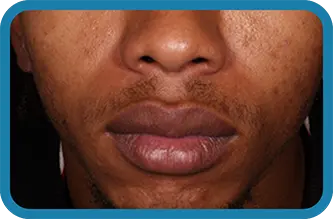
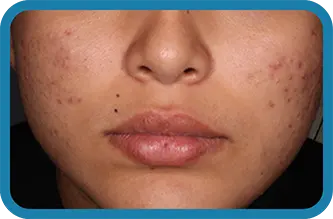
Actual clinical trial subject. Results may vary.

Actual clinical trial subject. Results may vary.
LOAD MORE

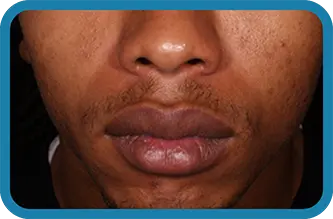
Actual clinical trial subject. Results may vary.
Actual clinical trial subject. Results may vary.

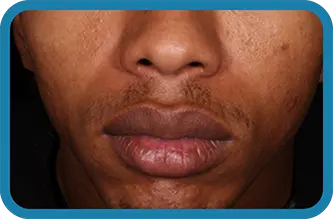
Actual clinical trial subject. Results may vary.
Actual clinical trial subject. Results may vary.
SHOW LESS
SEYSARA was effective WITH A DEMONSTRATED SAFETY PROFILE
-

GREATER REDUCTION IN INFLAMMATORY ACNE BY WEEK 12
-

IMPROVEMENT IN OVERALL ACNE SEVERITY BY WEEK 12
-

GENERALLY WELL TOLERATED BY PATIENTS
Reference:
1SEYSARA Package Insert. Almirall, LLC.